|
Badriprasad Ananthanarayanan B.S. Chem. Engr. (2002) Office : MRL 2027D, (805) 893 5658 |
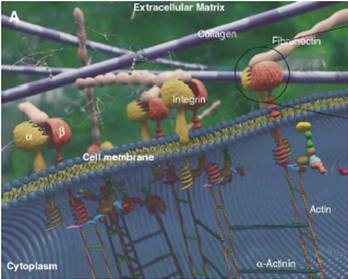
Cell adhesion to supported peptide-amphiphile bilayer membranes Our research focuses on understanding and manipulating the response of biological matter like human cells and blood to artificial materials. A wide variety of biomaterials are now being used clinically for various applications ranging from replacement limbs and organs to cardiovascular devices like stents and heart valves. The cell-material interface is also crucial to the performance of in vitro devices like biosensors. In all these scenarios it is of paramount importance to prevent negative reactions from the human body (e.g. the inflammatory response) which render the biomaterial ineffective. In many cases this can be best achieved by actively functionalizing biomaterials to induce a specific, positive response from the body. An intuitive strategy to achieve this is to engineer biomimetic systems, which take advantage of nature’s own recognition and response mechanisms. For instance, the extra-cellular matrix (ECM), which forms the natural environment of cells in human tissue, is known to harbor various proteins featuring adhesion sites that promote cell adhesion and proliferation (see Figure 1). These adhesive domains of the protein, i.e. short peptide ligands usually less than ten residues in length, are recognized by their counterpart receptor proteins on the cell surface. For example, the protein fibronectin in the ECM interacts with cell-surface integrins via two adhesive peptide domains: a minimal required sequence, RGD and a synergy site PHSRN. These strong and specific receptor-ligand interactions then trigger a sequence of intracellular events which eventually lead to stable adhesion, spreading and proliferation. Our approach to creating functionalized surfaces revolves around presenting these and other peptide ligands on surfaces in various controlled ways to induce the most favorable cellular response.
In order to accurately control the presentation of the peptide ligand at a surface, we use a synthetic construct known as a peptide amphiphile. Peptide amphiphiles (see Figure 2) consist of a peptide headgroup covalently linked, via an appropriate spacer, to a hydrophobic tail segment (usually two lipid-like hydrocarbon tails). The peptide headgroup gives the molecule its functionality while the hydrophobic tail segment drives its self-assembly with other hydrophobic molecules and amphiphiles into various structures including monolayers and bilayers, micelles, and vesicles. These amphiphiles can now be deposited onto surfaces by various self-assembly techniques, including Langmuir-Blodgett deposition and vesicle fusion. This allows us to create biofunctional surfaces with independent control over such parameters as the concentration and conformation and the functional peptide headgroup, as well as the accessibility of the ligand in terms of its distance from the surface (by varying the spacer length) and the spatial arrangement and mobility of the ligands in the surface-associated layer. These parameters can then be varied and the cellular response studied to derive insights into the mechanisms involved and the factors to be considered in the design of efficient functional biomaterials. |
|