Phase Transitions and Chemical Reactions at the Nano-Scale:
Effects of Confinement
Professor Keith Gubbins
Department of Chemical Engineering
North Carolina State University
Date:
Thursday, April 29, 2004
Time: 4:00 p.m.
Place: Engineering II, Room 3361
ABSTRACT
Nano-porous solids, such as zeolites, activated carbons, silicas and the recently synthesized mesoporous solids, have important applications in separations, catalysis, as sensors and in the synthesis of nano-structured materials. The behavior of phases confined within such nano-scale cavities can be strikingly different from that of the bulk material, due to finite size and confinement effects. Vapor pressures, diffusion rates, reaction yields and rates can all change by two orders of magnitude or more in small pores, and new phases and surface phenomena appear.
The influence of such confinement will be discussed for pores of simple geometry for phase transitions, with emphasis on freezing and melting. Both experimental and molecular simulation results will be presented; the results show qualitative agreement. Examples of the effects of confinement on reaction equilibria and reaction rates will also be discussed for simple geometries. Finally, some recent attempts to develop more realistic molecular models of disordered nano-porous materials will be described. Among the methods used to achieve this are mimetic simulation techniques, in which simulation protocols are developed to describe the synthesis of the material, and reconstruction methods which seek to construct models which match the experimental structural data.

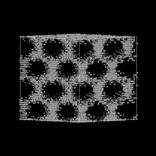
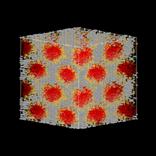
Mimetic simulation of MCM-41, a mesoporous silica material. (a) Hexagonal liquid crystal phase, (b) Surfactant and silica, (c) Silica porous structure. Tails are red, heads are yellow and silica is gray.