Research


|
Research

|
sd |
The Scott group conducts both fundamental and applied research in surface chemistry and catalysis. We aim to understand the interactions and transformations of molecules in solution and at gas-solid interfaces by creating highly uniform, grafted active sites. We apply techniques from organometallic
and coordination chemistry, surface science, spectroscopy, kinetics, mechanistic analysis and modeling to investigate, design and re-engineer heterogeneous catalysts. The group is comprised of researchers in chemistry and chemical engineering working together to solve problems at the interface of chemistry and reaction engineering. (download recent posters below) |
|
Redox Reactions Catalyzed by PGM-Substituted Complex Oxides |
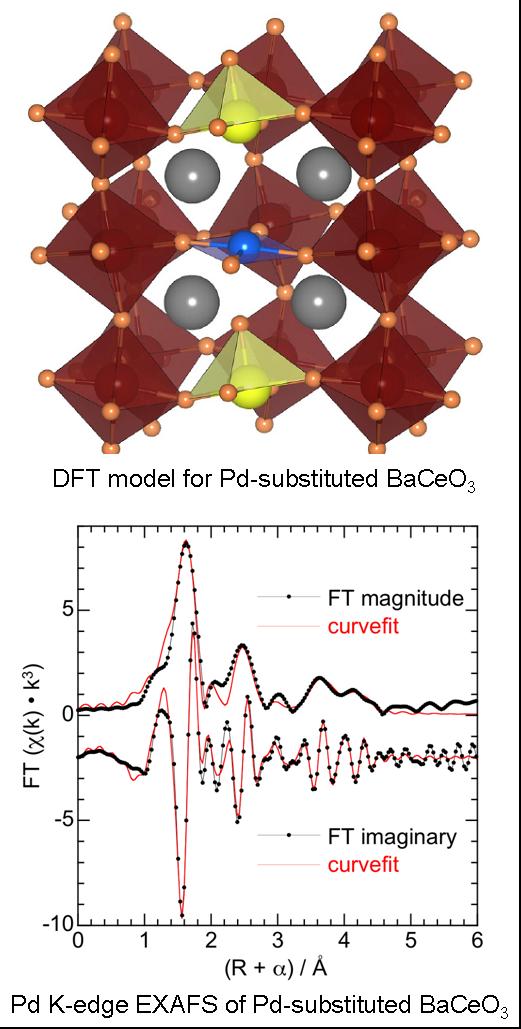 |
The incorporation of Pt-group metals (PGMs) into crystalline complex oxide hosts (e.g., the perovskite, in the example on the left) facilitates structural characterization and the direct comparison of experiment with theory. With these materials, we aim to tailor highly active and robust catalysts for the oxidation of CO and the reduction of NOx emissions in automotive exhaust streams. The low surface areas of perovskites do not preclude high catalytic
activity, and their high thermodynamic stability may be a significant benefit. Both BaO and CeO2 are stabilized against deactivation when combined as BaCeO3. Perovskite lattice (surface + bulk) oxygen promotes oxidation reactions at surface Pd sites. High lattice mobility is due to increased vacancy concentration associated with the presence of Pd(II) in the bulk. The CO oxidation mechanism is highly dependent on the CO concentration. At low P(CO), the reaction is strongly CO-inhibited. When high P(CO) causes nearly all the accessible Pd sites to be poisoned, a new mechanism involving lattice oxygen becomes available. This catalyst shows much higher activity than conventional catalysts such as Pd/Al2O3 under both types of reaction conditions. |
|
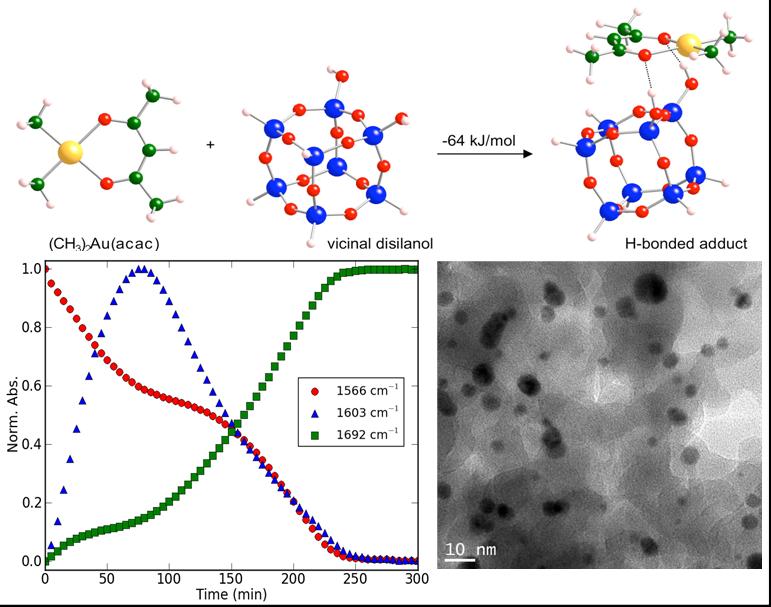
Controlled, Autocatalytic Synthesis of Metal Nanoparticles |
Metal nanoparticles dispersed on oxide supports show high activity and selectivity in oxidation, including low-temperature CO oxidation. We are exploring the scaleable synthesis of uniform, supported metal nanoparticles using molecular precursors. (CH3)2Au(acac) (acac = acetylacetonate) can be dispersed on oxides by chemical vapor deposition. With mild heating it is transformed to gold nanoparticles. The process can be monitored in situ by IR. Autocatalysis is clearly evident in the kinetics, providing fundamental information about the rates of particle nucleation and growth. By varying the support and the reaction conditions, we can establish the requirements for particle size selection.
|
 |
|
Alkene and Alkane Metathesis |
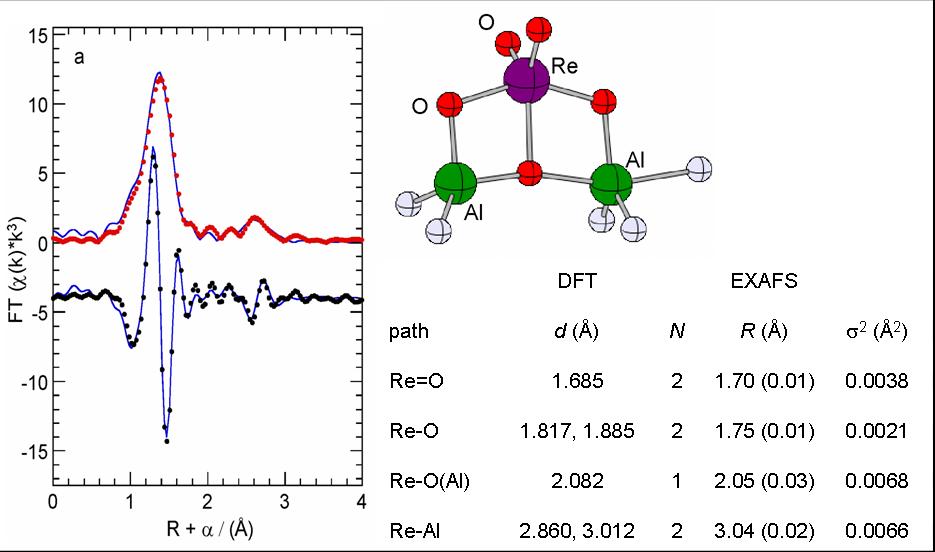 |
We aim to understand the activation and deactivation of supported olefin metathesis catalysts such as perrhenate (ReO4-) on alumina and methyltrioxorhenium (MTO) on silica-alumina, in order to develop rational ways to increase their productivity and extend the range of viable substrates to biomass-derived olefins such as unsaturated seed oils. Important questions include how the first metal-carbene active sites are formed, and how they interact with Lewis bases and polar molecules. An important application for this work is alkane metathesis, in which alkane dehydrogenation and olefin metathesis are coupled using a tandem catalyst system. It results in homologation of short-chain hydrocarbons so that they can be used as fuels (diesel-range). The lifetime of the olefin metathesis catalyst is key.
|
|
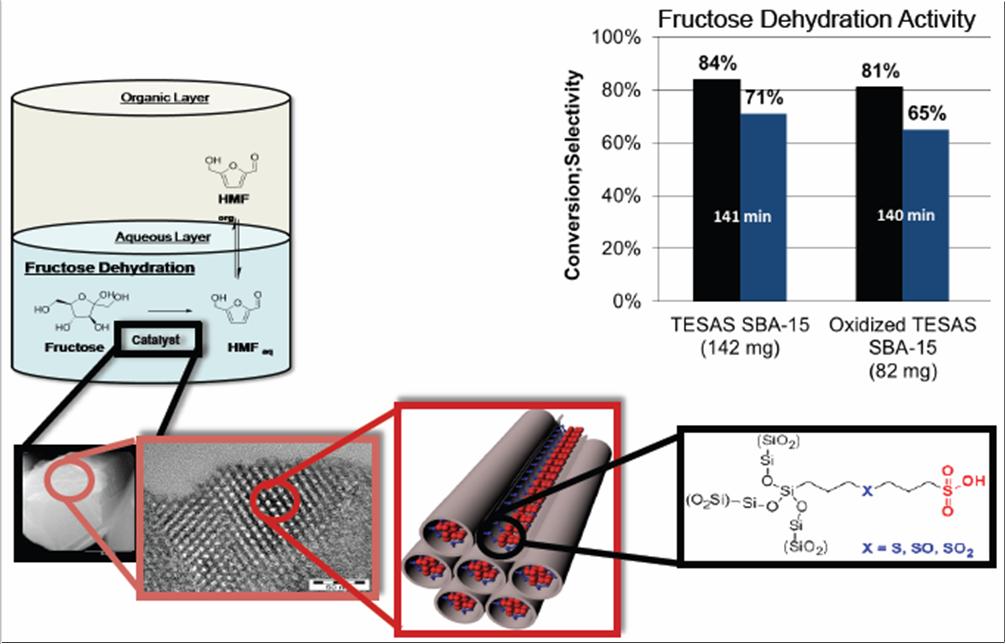
New Catalysts for Converting Biomass to Fuels and Chemicals |
This project targets the development of solid catalysts for the efficient conversion of non-food biomass feedstocks (lignocellulose and soluble carbohydrates) to fuels and platform chemicals. One such reaction is the dehydration of sugars to 5-hydroxymethylfurfural (HMF), for which we designed an ordered mesoporous acid catalyst. The tandem hydrogenation or oxidation of HMF using a second, supported catalyst is underway.
|
 |
|
Supported Olefin Polymerization Catalysts |
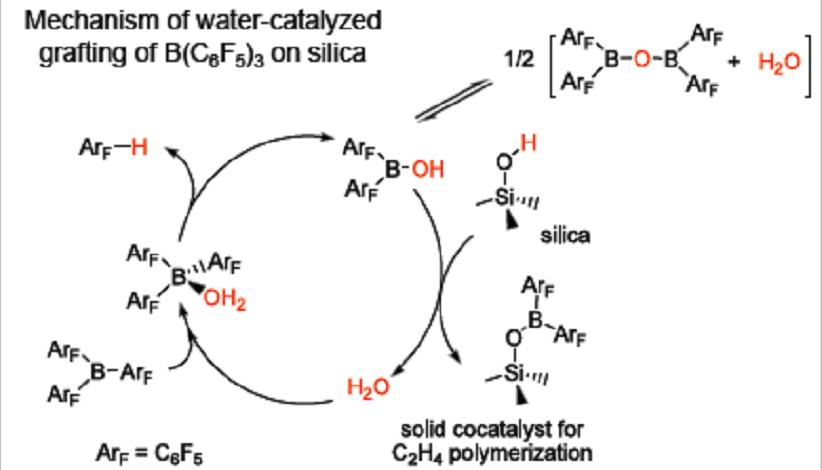
We investigate the relationships between active site structures and polymerization activity for heterogeneous Phillips (Cr) and Ziegler-Natta (Ti,V) catalysts, as well as supported single-site catalysts. Frequently, the catalystsupport interaction is crucial to the appearance of activity. Grafting of B(C6F5)3 onto silica to create a robustlyanchored co-catalyst for single-site olefin polymerization has been achieved for the first time in our lab. The reaction is catalyzed by trace water, which can be generated in situ by borane-induced dehydroxylation of surface silanols, or added intentionally. The grafted borane is a well-defined, Lewis acid that activates molecular complexes of Cr and Ni for olefin polymerization.
|
 |
|
Spatial Distribution of Grafting Sites on Silica |
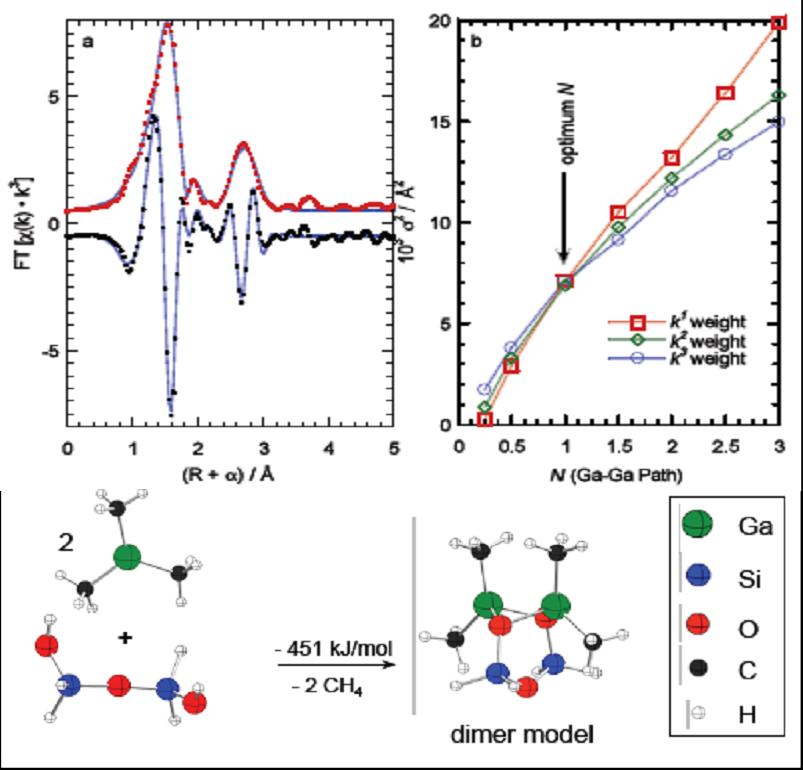 |
Amorphous silica is an important support for many heterogeneous catalysts. It also has one of the simplest oxide surfaces, possessing only weakly Bronsted acidic hydroxyl groups, siloxane bonds and no Lewis acid sites. The hydroxyl groups represent the principal grafting sites for molecular catalysts. It is widely assumed that they become isolated during thermal pretreatment of the silica. We probed the distribution between the hydroxyls by studying their interaction with Ga(CH3)3. Amorphous silica is an important support for many heterogeneous catalysts. It also has one of the simplest oxide surfaces, possessing only weakly Bronsted acidic hydroxyl groups, siloxane bonds and no Lewis acid sites. The hydroxyl groups represent the principal grafting sites for molecular catalysts. It is widely assumed that they become isolated during thermal pretreatment of the silica. We probed the distribution between the hydroxyls by studying their interaction with Ga(CH3)3.
|
|
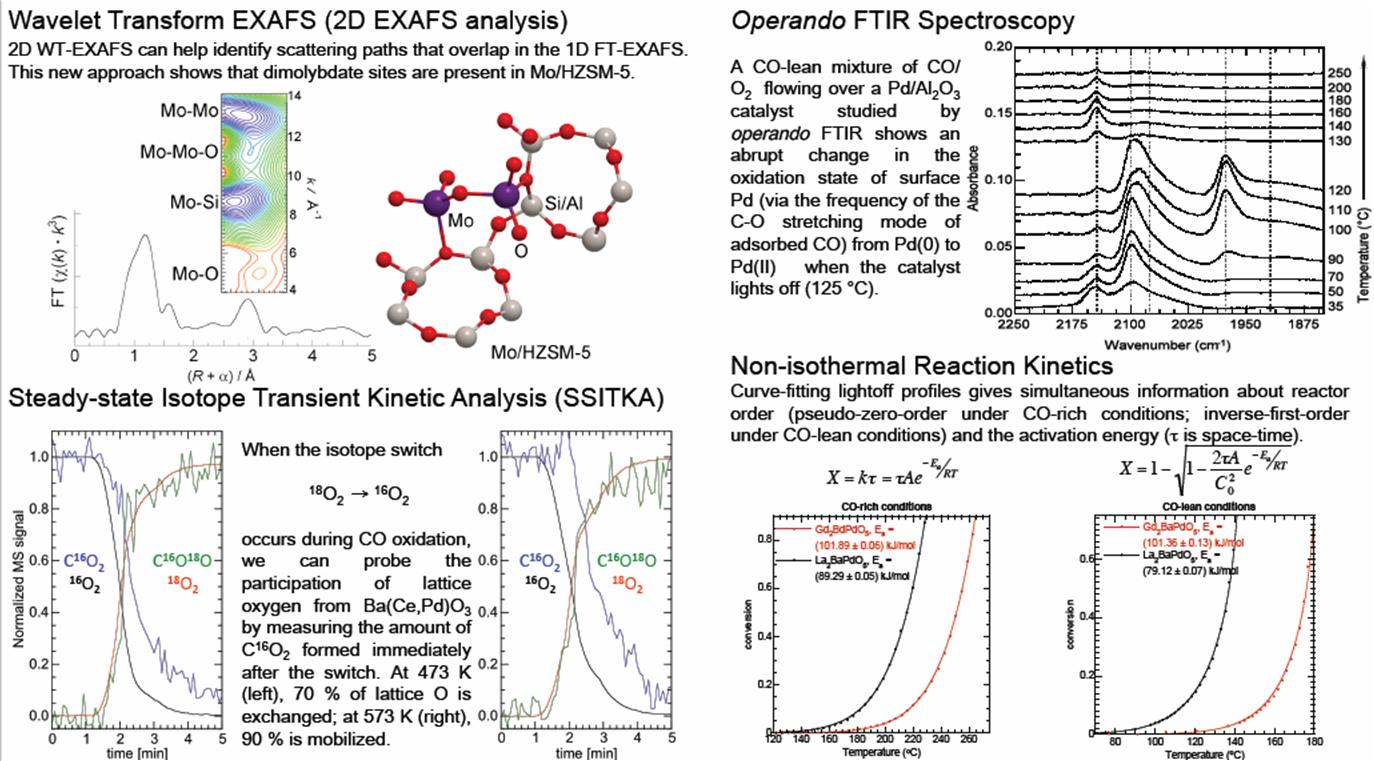
New Spectroscopic and Kinetic Methods for Catalysis |
 |
|
Posters and Covers |
|
|